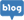Product
development
inquiry
Novarex
Official Blog
R&D Center
The Research and Development Center (R&D) develops excellent functional ingredients for health functional foods through
raw material standardization, safety evaluation and functional (preclinical and human study)
research using various ingredients such as naturally-derived animals and plants and microorganisms.

Functional materials developed based on scientifically verified research results realize the era of Healthcare 3.0, which is to extend the lifespan of human health through securing intellectual property rights and global licensing of materials.
Business Introduction
In order to discover various functional materials, the Novarex R&D Center is continuously developing excellent functional materials by evaluating the efficacy and safety through cell/animal testing as well as human studies.
Sourcing of functional ingredients
Discovering new ingredients at home and abroad
Material R&D
Joint research with domestic and foreign partners
Licensed in leading institutions

Raw material standardization
Standard Operating Procedure (SOP)
Establishment of raw material standard and test methods (Certificate of Analysis, CoA)
Method Validation
Hazardous substance standard setting
Food Standard Specification Review

Safety study
Domestic and international recognition and product status
Establishment of intake evaluation data
GLP toxicity test
Functional study
Preclinical efficacy verification test
(cell/animal test)
Functional mechanism study
Design and conduct human research

Licensing
(individually recognized)
Review and write individual recognition application materials
Application for individual approval
Group discussion, product briefing session
Approval for functional ingredients

Global Licensing
U.S(FDA)
Europe(EFSA)
Canada(Health Canada)
Australia(TGA)
China(CFDA)
Japan(JFDA)
Construction of global marketing materials
R&D cooperation system
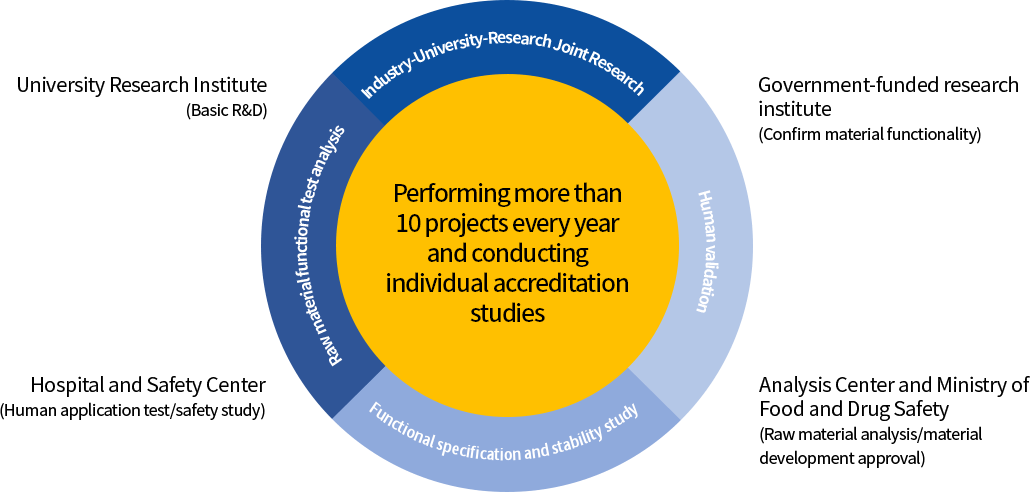
| University Research Institute |
|
|---|---|
| Government-funded research institute |
|
| Institutions performing human application tests |
|
| Certified Analytical Institutions and GLP Institutions |
|
 Functional ingredients
Functional ingredients